Why participate in a clinical trial?
Participation in clinical trials is essential for the progress of medicine by contributing to the development of more effective and safer treatments.
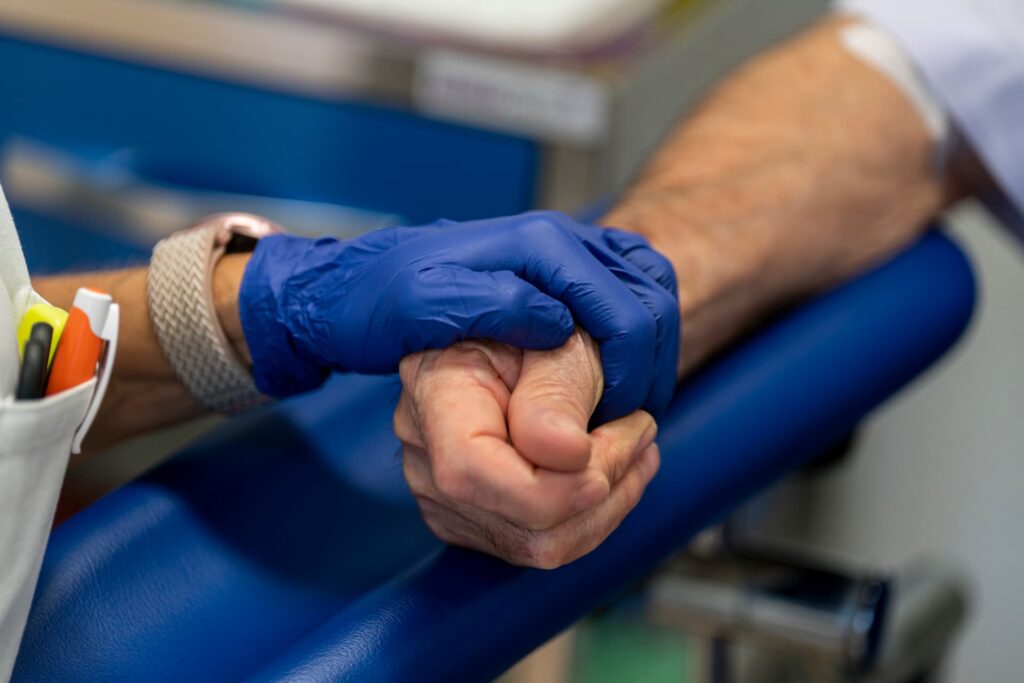
Platform for recruiting volunteers interested in participating in clinical trials aimed at improving medical research and global health.
We connect people interested in contributing to scientific advancement with studies evaluating new treatments and therapies. Volunteers can personally benefit and be part of medical progress by participating in trials that address a variety of conditions.
A clinical trial is a research study where volunteer individuals participate to test potential new treatments with the aim of determining their safety and efficacy.
Eligibility to participate in a clinical study depends on several factors and may vary according to the design and specific objectives of each study. Inclusion and exclusion criteria are designed to ensure the safety of participants and the validity of the results. Some criteria include: medical diagnosis, age, health status, medical history and previous treatments, among others.

Participation in clinical trials is essential for the progress of medicine by contributing to the development of more effective and safer treatments.
The main objective is to evaluate safety in humans and guide the most appropriate dosage and administration schedule.
When a person decides to participate in a clinical study, they are guaranteed a series of rights and also assume various responsibilities that are fundamental to the success and integrity of the research.
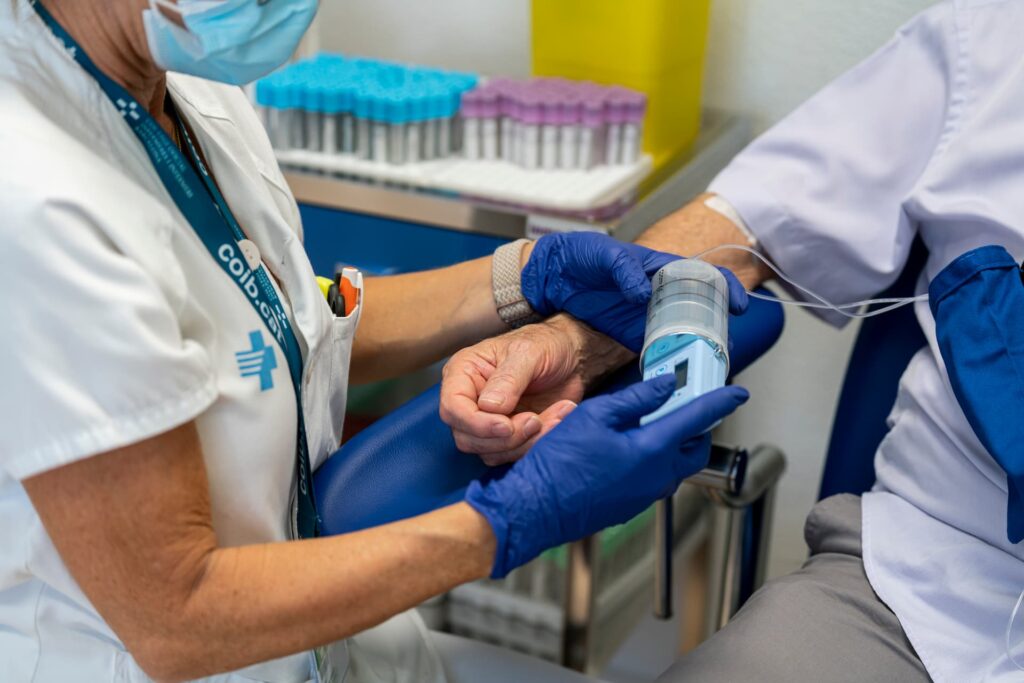
The decision to participate in a clinical study is personal and should be made voluntarily, without external pressures. Whether individuals are in good health or have a medical diagnosis, they should engage in clinical research only if they feel well-informed and comfortable with the entire process.
We offer you a guidance guide so that you are aware of the important questions before joining a study. We understand that deciding to participate in a study is a significant step, and our goal is to provide you with all the necessary information. Below, you will find some essential questions that will help you make an informed decision: