Feedback
What do they think of us?


We promote research by offering comprehensive support in the management and implementation of clinical studies.
We provide advice, solutions and structures that accompany the investigator throughout the clinical study process, from design to execution, guaranteeing compliance with all ethical and regulatory standards and ensuring the quality and validity of the research.
Our success lies in the combined efforts from the CRO and CTU teams that provide thorough support to the research team.

What do they think of us?
Check out some of the latest projects we’ve worked on.
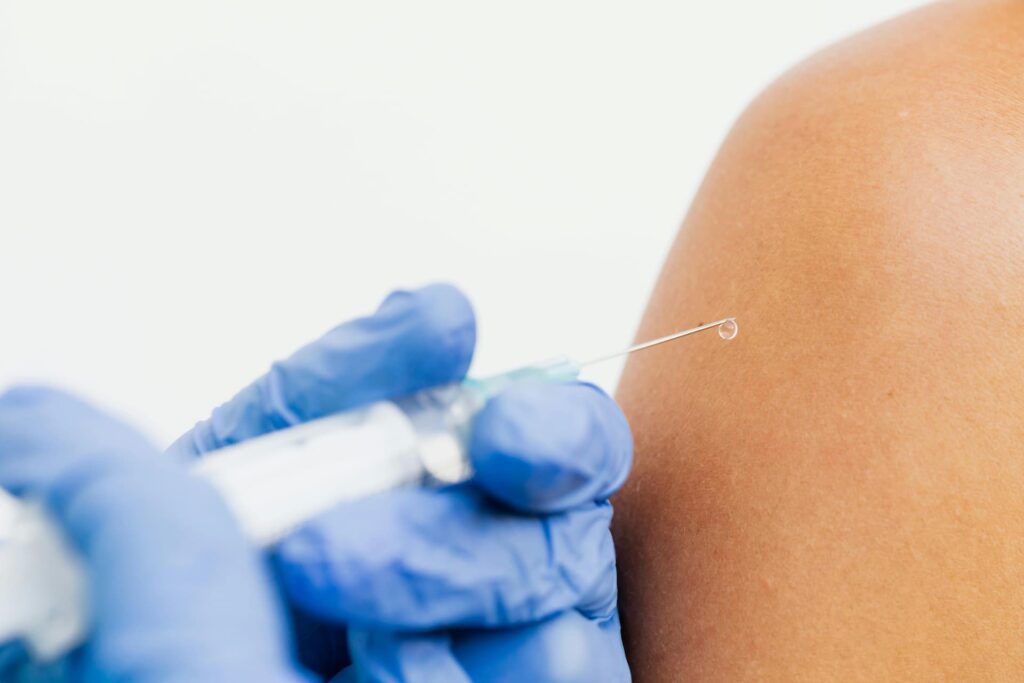
The HOLA trial seeks to evaluate the feasibility of administering cabotegravir and rilpivirine intramuscularly in extrahospital centers in Spain for HIV patients. Currently in the recruitment phase, over 50% of the anticipated participants have been randomized across various hospitals and community centers. The research team, in collaboration with ScienHub Research Support, is overcoming obstacles and identifying facilitating factors, aiming to determine the viability of this intramuscular administration outside the hospital setting.
The SCHISTO-STOP study, which focuses on implementing a schistosomiasis detection strategy among immigrant populations at epidemiological risk, has yielded encouraging preliminary results. Collaboration between researchers and ScienHub Research Support teams has enabled the study to be completed as planned, yielding promising results that could have a significant impact on global strategies to improve the quality of life for this vulnerable population group.

Currently, ScienHub Research Support is offering assistance for two clinical trials underway in Spain under the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT). ScienHub Research Support’s CTU is responsible for orchestrating the study’s implementation at the HUGTiP. Their duties include executing visit procedures meticulously outlined in the protocol. Simultaneously, ScienHub Research Support’s CRO is diligently handling bureaucratic procedures and providing crucial management support. Furthermore, they are managing monitoring activities at various participating centers scattered throughout Spain.